structure
determination of bacteriophage endolysins

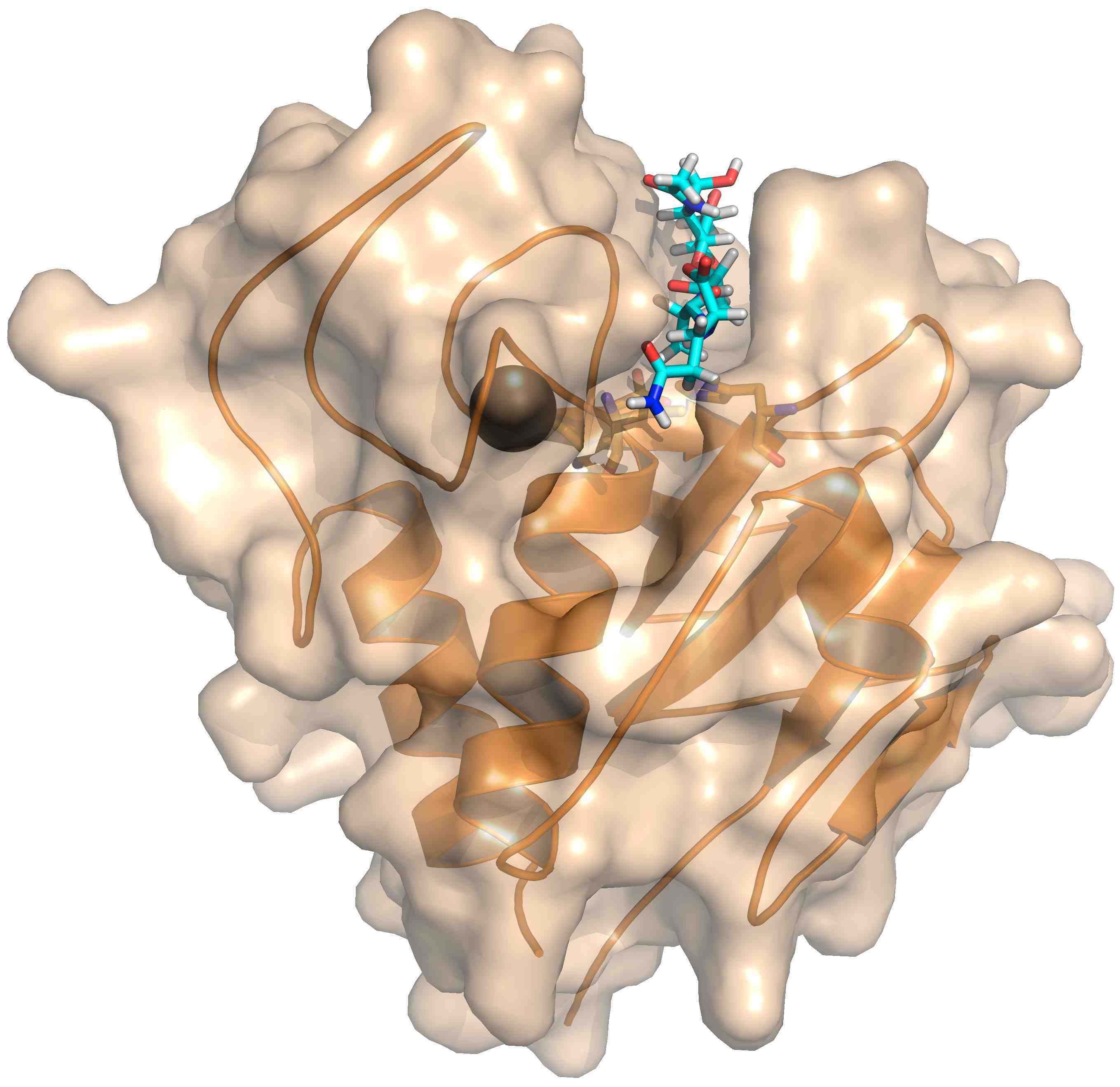
To be released from the infected bacterial cell, phages express lysis proteins. Holins make holes in the internal membranes, while endolysins digest the peptidoglycan layer. In the case of Gram-positive bacteria, endolysins are also effective when applied externally. Therefore, we have started crystallographic studies of endolysin active domains and have recently solved the structure of the CHAP domain of the endolysin of the staphylococcal phage K. It contains a calcium ion near the active site which is important for activity.
publications
-
Perczel A, Atanasov AG, Sklenář V, Nováček J, Papoušková
V, Kadeřávek P, Žídek L, Kozłowski H, Wątły J, Hecel A,
Kołkowska P, Koča J, Svobodová-Vařeková R, Pravda L,
Sehnal D, Horský V, Geidl S, Enriz RD, Matějka P,
Jeništová A, Dendisová M, Kokaislová A, Weissig V, Olsen
M, Coffey A, Ajuebor J, Keary R, Sanz-Gaitero M, van
Raaij MJ, McAuliffe O, Waltenberger B, Mocan A, Šmejkal
K, Heiss EH, Diederich M, Musioł R, Košmrlj J, Polański
J, Jampílek J (2016) The Eighth Central European
Conference "Chemistry towards Biology": Snapshot.
Molecules 21, E1381.
- Keary R, Sanz-Gaitero M, van Raaij MJ,
O’Mahony J, Fenton M, McAuliffe O, Hill C, Ross RP,
Coffey A (2015). Characterization of a
bacteriophage-derived murein peptidase for elimination
of antibiotic resistant Staphylococcus aureus. Current
Protein Pept Sci 17, 183-190.
- Sanz-Gaitero M, Keary R, Garcia-Doval C, Coffey A, van Raaij
MJ (2014). Crystal structure of the lytic CHAPK domain of the
endolysin LysK from Staphylococcus aureus bacteriophage K.
Virol J, 133.
- Sanz-Gaitero M, Keary R, Garcia-Doval C, Coffey A, van Raaij
MJ (2013) Crystallization of the CHAP domain of the endolysin
from Staphylococcus aureus bacteriophage K. Acta Cryst F69,
1393-1396.
back home